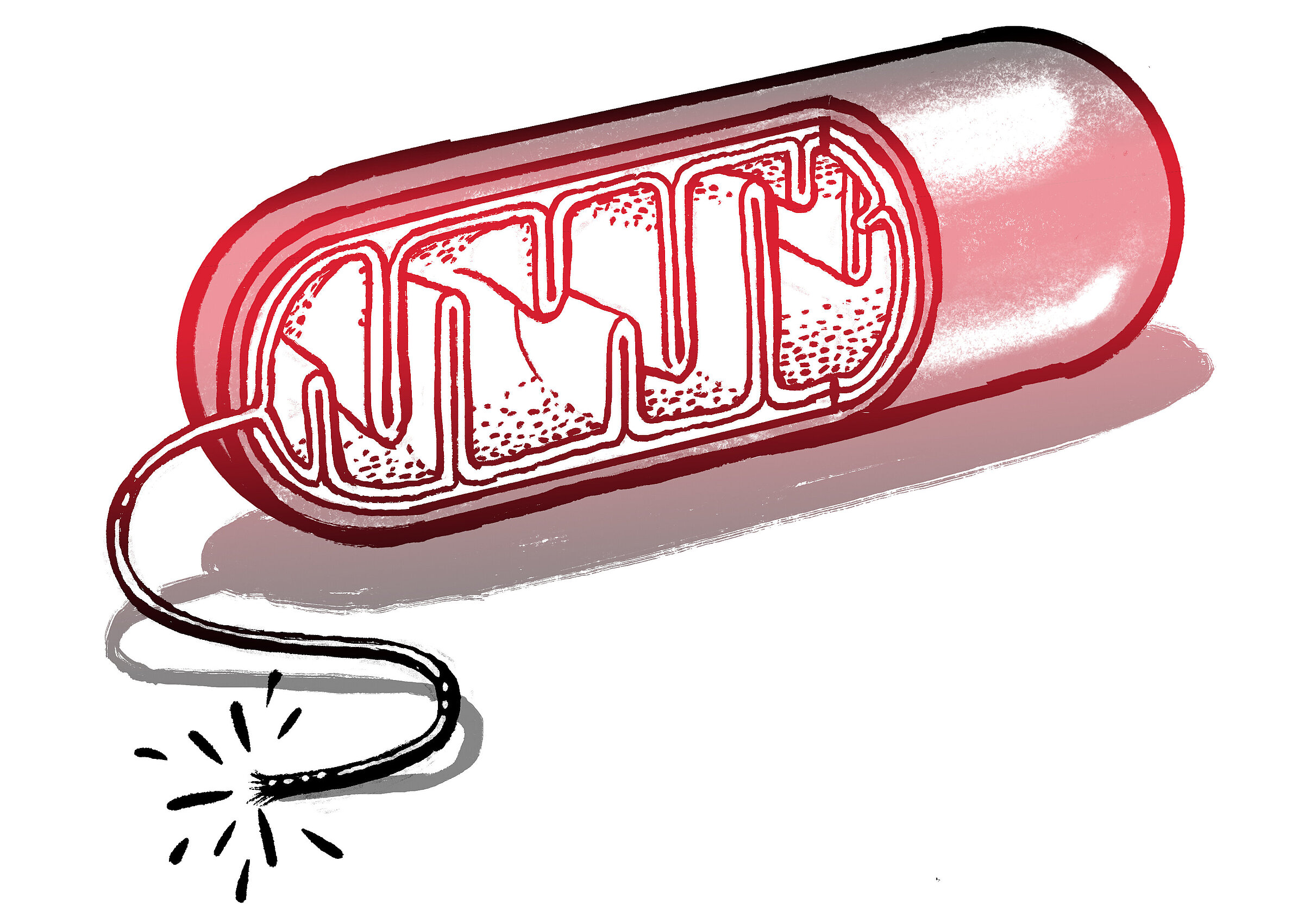
Mitochondria are effective energy producers for cells. They essentially function like fuel cells in which electrons are transported across a membrane to generate energy. But instead of hydrogen and oxygen, mitochondria require sugar and oxygen. They also act as a kill switch, collecting signals and helping to “decide” whether a cell commits suicide. This happens when certain enzymes, acting as a sensor, spread through the mitochondria and send a signal that something bad has happened that can’t be repaired. An example would be an interruption to the sugar supply. The mitochondria then trigger a self-destruct sequence called apoptosis or programmed cell death.
Apoptosis has important biological functions. For instance, it coordinates the death of certain cells during human development. If this didn’t happen, adults would still have webbed fingers and toes, among other things. However, misdirected apoptosis can have dramatic consequences, leading, for example, to the development of tumors or dementia. When someone suffers a stroke, cell death leads to paralysis or speech problems.
I study diseases of the mitochondria. These are very rare diseases characterized, among other things, by defective glucose metabolism in mitochondria. They occur primarily in metabolically active tissue, such as brain tissue. The symptoms can vary considerably. Some patients experience repeated strokes, while others suffer nerve damage. Until now, it has been almost impossible to treat them.
I want to understand the biochemical mechanisms that lead to cell death and develop treatments for mitochondrial diseases. In order to do this, we are creating organoid models in the lab. We are generating brain-like tissue derived from stem cells that fulfills the brain’s core functions and presents patient-specific mutations. Using these organoids, we try to understand how mutation loads affect glucose metabolism and cell death. Which mechanisms cause a nerve cell to die in certain stress situations? And what are the possible ways to prevent this from happening? We want to test approved drugs on the organoid to see if they can prevent cell death. We are also in the process of introducing a method based on magnetic resonance imaging that allows us to measure whether these drugs also affect cell metabolism in the brains of patients.
The expectation is that the findings from my research will be transferrable to conditions such as strokes and neurodegenerative diseases. In a stroke, blood clots prevent enough oxygen and glucose reaching the nerve cells, which start to die after just a few minutes. The only treatment currently available is to remove the blood clots and reestablish blood flow, which is only possible in around 15 percent of patients. There is still no treatment that targets the regulation of cell death or metabolism. I hope that my research will help find mechanisms that can delay or prevent cell death and minimize the damage to brain tissue.
I am fascinated by the biological functions behind diseases. But as a physician-scientist, I also see it as my duty to bring research findings to the people who need them. Back when I was studying, neurology had the reputation of not being able to do much for patients. The situation today is completely different and there are plenty of new treatments in the pipeline. For me, working with patients, treating them while advancing research, is both a great privilege and a very emotional job.